Effect of toxic metals on the bone regeneration
Vliv toxických kovů na regeneraci kostí
Úvod: V současné době může velmi často docházet k hojení zlomenin kostí v podmínkách nadměrného příjmu olova a kadmia, protože tyto prvky patří mezi deset nejčastějších znečišťujících látek v životním prostředí. Zároveň v odborné literatuře chybí práce věnované kombinovanému subchronickému účinku nadměrného množství olova a kadmia na strukturu přímých činitelů reparační osteogeneze – buněk regenerujících kost. Cíl: Zjistit subchronický účinek nadměrného množství olova a kadmia na mikro- a ultrastrukturu buněk regenerujících kost. Materiál a metody: Pokus byl proveden na 24 bílých potkanech Wistar, kteří byli rozděleni do 2 skupin. Jedinci skupiny I konzumovali pitnou vodu standardní kvality a jedinci skupiny II dostávali po dobu 3 měsíců žaludeční sondou vodu se směsí dusičnanu olovnatého a chloridu kademnatého v dávce 87,74 mg/kg a 2,14 mg/kg. U všech jedinců byl 24 a 3 dny před koncem 3měsíčního experimentu ve střední části diafýzy tibie vytvořen otvor o průměru 1,5 mm zasahující až do dutiny dřeňové. Studium mikro- a ultrastruktury buněk kostní regenerace bylo provedeno pomocí skenovací a transmisní elektronové mikroskopie. Výsledky: Bylo zjištěno, že v podmínkách subchronického příjmu nadměrného množství olova a kadmia byly v regenerovaných lymfocytech pozorovány velké perinukleární prostory a prosvětlení cytoplazmy, osteocyty regenerované kostní tkáně měly převážně krátké výběžky a v osteoblastech byly elektronově průhledné cisterny granulárního endoplazmatického retikula, invaginace jaderné membrány, oblast elektronické průhlednosti matrix a lýza krystaly mitochondrií. Závěr: Subchronický příjem nadměrného množství olova a kadmia v organizmu vede k dystrofickým a destruktivním změnám buněčných elementů kostního regenerátu a zpomalení jejich zrání.
Authors:
Korenkov Oleksii; Larina Kateryna
Authors place of work:
Sumy State University, Sumy, Ukraine
Published in the journal:
Clinical Osteology 2023; 28(4): 118-124
Category:
Původní články
Summary
Background: At present, the healing of broken bones can very often occur in conditions of excessive intake of lead and cadmium, as these elements are among the ten most common environmental pollutants according. At the same time, there are no works in the scientific literature devoted to the combined subchronic effect of excessive amounts of Pb and Cd on the structure of the direct performers of reparative osteogenesis – bone regenerate cells. Aim: To establish the subchronic effect of excessive amounts of lead and cadmium on the micro-and ultrastructure of bone regenerate cells. Material and methods: The experiment was conducted on 24 white Wistar rats, which were divided into 2 groups. Group I animals consumed drinking water of standard quality, and group II animals received water with a mixture of lead nitrate and cadmium chloride dissolved in it at a dose of 87.74 mg/kg and 2.14 mg/kg through a gastric tube for 3 months. For all the animals 24 and 3 days before the end of the 3-month experiment in the middle of the tibial diaphysis there was reproduced a hole defect with a diameter of 1.5 mm to the bone marrow canal. The study of micro- and ultrastructure of bone regenerate cells was performed using scanning and transmission electron microscopy. Results: It was found that under the conditions of subchronic intake of excessive amounts of lead and cadmium in the regenerate lymphocytes, large perinuclear spaces and cytoplasm enlightenment were observed, osteocytes of the regenerate bone tissue had mainly short processes, and in osteoblasts there were electron-transparent cisterns of the granular endoplasmic reticulum, nuclear membrane invagination, an area of electronic transparency of the matrix and lysis of mitochondria cristae. Conclusion: Subchronic receipt of excessive amounts of lead and cadmium in the body leads to dystrophic and destructive changes in the cellular elements of bone regenerate and slowing their maturation.
Introduction
The stability of the elemental composition of the body is one of the most important and mandatory conditions for normal bone regeneration. This is due to the fact that micro-and macroelements are components of enzymes and proteins that are involved in stopping bleeding during trauma, proliferation and differentiation of cellular elements, angiogenesis, oxygenation of regenerate cells, synthesis of components of the organic matrix of bone and its mineralization, energy formation and protection of cells from free radicals [1–3]. However, to perform these functions, each element has an optimal range of concentrations, a decrease or increase in which can lead to pathological changes [4].
The intensification of technogenesis as a characteristic feature of the 3rd Millennium may be one of the reasons for the violation of homeostasis of chemical elements in the body and, as a result, the regeneration of bone tissue [5–7]. At the same time, we note that of all the inorganic compounds that enter the biosphere as a result of human activity, lead and cadmium are among the ten most dangerous according to the world health organization. The latter continue to accumulate in the environment and enter the human body in excess. For example, in Ukraine, sanitary and toxicological studies have found that samples of drinking water from agricultural and processing enterprises of the agro-industrial complex contain Pb (0.1 mg/l) and Cd (0.035 mg/l) exceeding the maximum permissible concentrations from 10 to 35 times [8]. The latter indicates that for the people who live in these territories, healing of broken bones can very often occur in conditions of consumption drinking water, which does not meet hygienic requirements. Note that the reported concentrations of lead and cadmium in drinking water are relatively small compared to their average lethal doses. According to Lu HK and co-authors, the average lethal dose of lead and cadmium for rats is 2 633 mg/kg and 64 mg/kg, which is much higher than the content of these heavy metals in the drinking water of the agro-industrial complex of Ukraine [9]. Therefore, probably the greatest severity of negative effects from such concentrations of lead and cadmium can be manifested during their long-term subchronic or chronic intake into the body. It should also be noted that there is evidence in the literature regarding a separate effect of increased concentrations of lead and cadmium on bone regeneration [10–13]. The results of these studies show that the excess of lead delays the healing process of bone fractures, suppresses the mineralization of bone corns, and cadmium reduces the expression of genes that are involved in the differentiation of osteoblasts, the activity of the enzyme alkaline phosphatase, the mineralization of regenerative bone tissue, delays the growth, density and biomechanical properties of bones [3,14–17]. The mechanism of this effect is due to the ability of heavy metals to reduce the antioxidant potential of the body, generate reactive oxygen species, displace and replace essential micro – and macronutrients in the molecules of enzymes and other biologically active substances, as well as to suppress signaling systems that activate the process of differentiation of cellular elements of bone tissue [18–26]. During the review of the literature, we also found works on the combined effect of excess lead and cadmium on the microscopic structure of bone tissue and its physiological regeneration [9,27,28]. However, we have not found work on the combined effect of excessive amounts of lead and cadmium on the micro-and ultramicroscopic structure of the direct performers of reparative osteogenesis – bone regenerate cells.
So, the purpose of our work was to establish the subchronic effect of excessive amounts of lead and cadmium on the micro-and ultrastructure of bone regenerate cells.
Material and methods
Animals
An experimental morphological study was conducted on 24 Wistar rats with a weight of about 300 grams. Animal experiments were approved by the Commission on biomedical ethics of Sumy state University (Minutes № 1/1 of 16.01.2023) and were performed in accordance with the rules of the “European Convention for the protection of vertebrates used for experimental and other scientific purposes”. Throughout the experiment, the animals were properly cared for and kept on a standard diet and had free access to drinking water.
All the animals were divided into 2 groups
Group I (12 rats) – control animals that consumed drinking water of standard quality [29]
Group II (12 rats) – animals that were administered 4 ml of water with a mixture of lead nitrate (Pb(NO3)2) and cadmium chloride (CdCl2•2.5H₂O) from Sigma-Aldrich with a dose of 87.74 mg/kg and 2.14 mg/kg through a gastric tube for three months. The choice of this dose was based on studies by Lu HK and co-authors, who studied the experimental acute combined effect of lead and cadmium on bone damage in rats and established their average lethal dose of LD50 (2 633 mg/kg for lead and 64 mg/kg for cadmium) [9]. In our experiment, the dose of lead and cadmium was 1/30 of the LD50, which, according to Lu HK and co-authors, corresponds to a subchronic toxicological effect on the rat body after three months of use [27].
Surgical intervention
24 and 3 days before the end of the three-month consumption of water with heavy metal salts by animals in the operating room under intramuscular ketamine anesthesia (50–75 mg/kg, Calypsol: Gedeon Richter, Budapest, Hungary), observing aseptic and antiseptic conditions, surgical intervention was performed. Before the operation, the rats were fixed on a Board in a position on their back and the wool was cut off on their shins. The operating field was treated with 3% alcohol solution of iodine. Then there was made a longitudinal incision of the skin and fascia along the line of margo anterior tibia, took them aside and exposed the tibial diaphysis. In the middle of the diaphysis, the defect was applied to the bone-marrow canal using a drill (Korund-NX, VIOLA, Kiev, Ukraine) with a ball mill with a diameter of 1.5 mm at low rpm and under jet cooling with saline solution. The place of application of the defect was chosen taking into account the least traumatization of soft tissues. The operating wound was sutured with a catgut suture.
Animals were removed from the experiment by decapitation under deep ketamine anesthesia (100 mg/kg) on the 3rd and 24th day from the moment of injury. Scanning and transmission electron microscopy was used to study the micro- and ultrastructure of bone regenerate cells.
Scanning electron microscopy
To study the cell elements of the regenerate by scanning electron microscopy, fragments of the tibia with the injury site were fixed in a 2.5% buffer solution of glutaraldehyde for 24 hours. Then they were washed with a buffer solution and placed for final fixation in a 1 % buffer solution of osmium tetrachloride for 1 hour. Dehydration was performed in alcohols of increasing concentration and acetone. Bone samples were glued to metal tables with conductive glue, filed with carbon in a standard vacuum unit of the VUP-5 type (Selmi, JSC, Sumy, Ukraine) and examined with a scanning electron microscope “REM 106-I” (Selmi, JSC, Sumy, Ukraine).
Transmission electron microscopy
To study the cell elements of the regenerate by transmission electron microscopy, a part of the bone marrow was cut out and fixed in a 2.5 % buffer solution of glutaraldehyde for 24 hours. Decalcification was performed in a solution of Trilon B at a temperature of 4 °C. After decalcification, the tissue pieces were washed with a buffer solution and placed for final fixation in a 1% buffer solution of osmium tetroxide for 1 hour. Dehydration was performed in alcohols of increasing concentration and acetone. Then the pieces of tissue were soaked in a mixture of epoxy resins (EPON-Araldite). Polymerization of the blocks was carried out in a thermostat at a temperature of 60 °C for 2 days. Ultrathin sections were made on ultramicrotom UMTP-6M (Selmi, JSC, Sumy, Ukraine), mounted on electrolytic grids, which after contrasting with lead citrate were studied under an electron microscope EMV-100BR (Selmi, JSC, Sumy, Ukraine).
Results
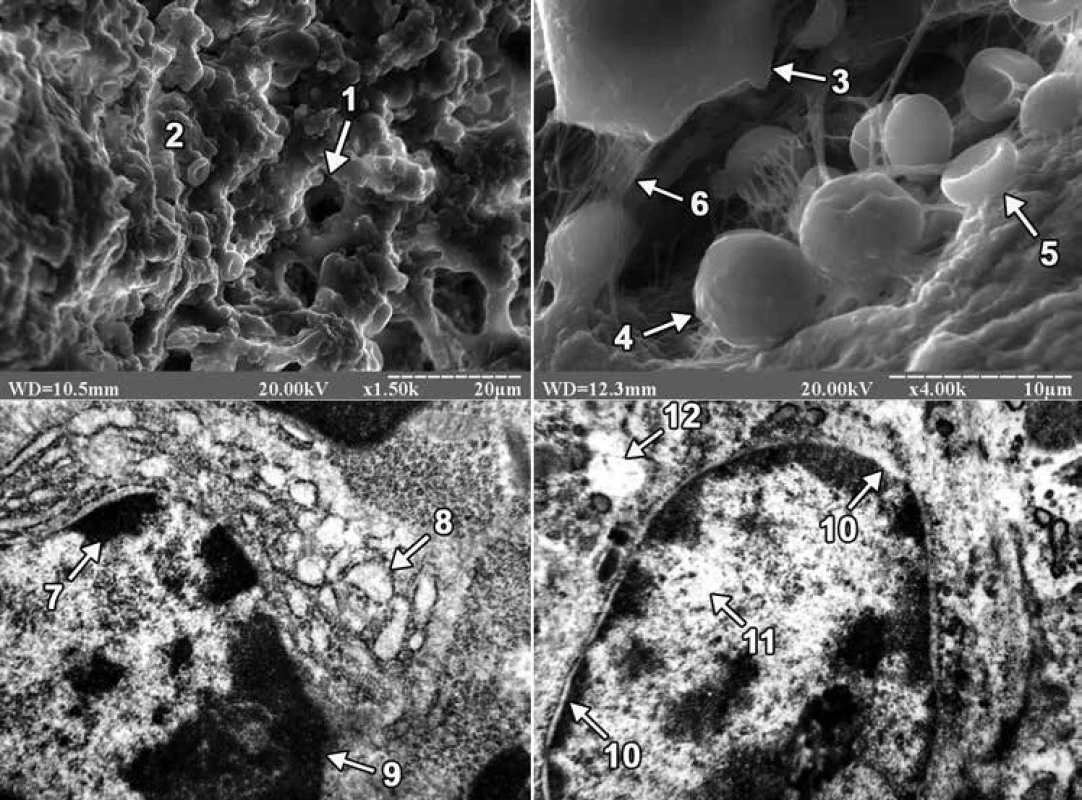
On the 3rd day after the injury in animals of both groups, granulation tissue and a conglomerate of cells that had morphological features of neutrophils, platelets, red blood cells, osteogenic cells, macrophages and lymphocytes were found in the area of the bone defect when viewed on a raster electron microscope. However, in ultrathin sections of regenerate in the animals of both groups, lymphocytes were mainly detected. In animals of the first group, the nuclei of lymphocytes occupied a significant part of the cytoplasm, the nuclear membrane was smooth, and the perinuclear spaces were not expanded. On the periphery of the core matrix in the form of large osmiophilic depressions there were localized granules of condensed chromatin, and in the Central region of the core of karyoplasm had a low electronic density and contained a small number of granules of decondensed chromatin. The well-developed granular endoplasmic reticulum, whose cisterns were significantly expanded, and numerous ribosomes were detected on the membranes, also attracted attention. At the same time, in animals of the second group, the nuclei of lymphocytes were also large, containing mainly condensed chromatin, and the granules of decondensed chromatin were located in the Central region of the nucleus. The core matrix had a low electron density, and the perinuclear spaces, unlike the animals of the first group, were greatly expanded. Lymphocytes of animals of the second group were also characterized by the presence of an enlightened cytoplasm, which contained separate cisterns of the granular endoplasmic reticulum (fig. 1).
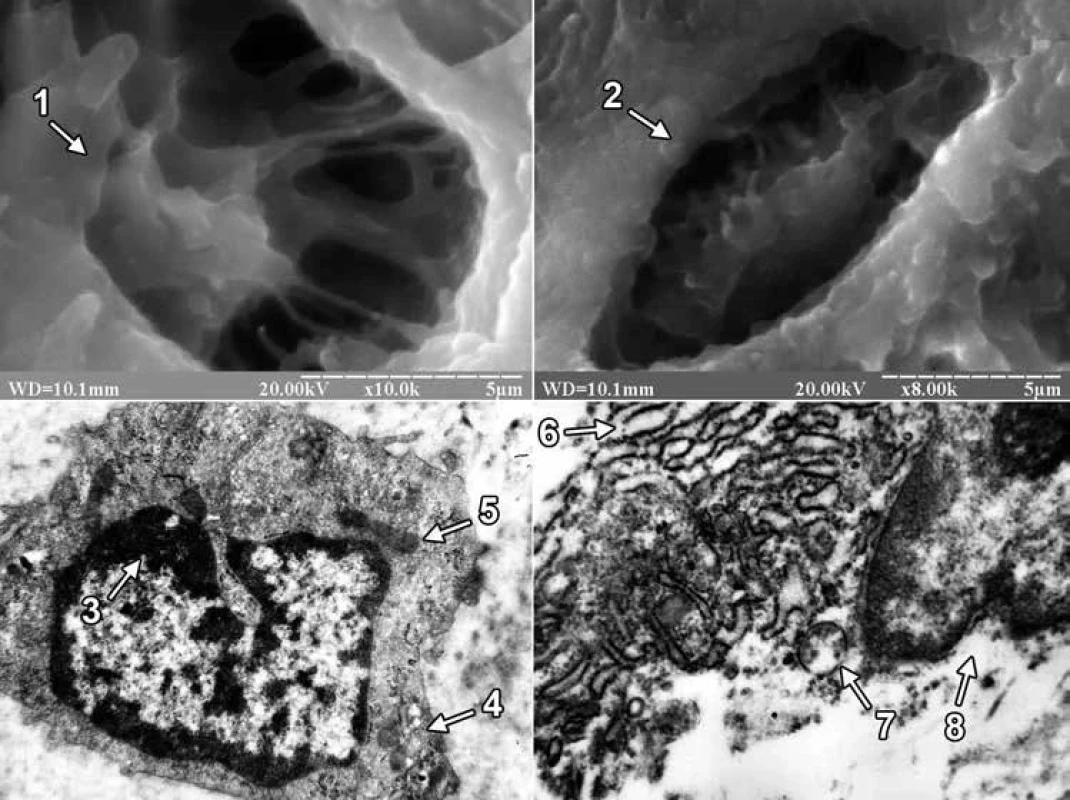
On the 24th day of the experiment, the site of the bone defect was filled with regenerate bone tissue, which included primary and secondary osteoblasts in both groups of animals. Primary osteoblasts were located on the surface of the trabeculae of the regenerate bone tissue, and secondary osteoblasts created bone plates and turned into secondary osteocytes. At the same time, the difference in the structure of the external surface of osteocytes between the animals of the experimental groups attracted attention. The latter consisted in the fact that in the first group of animals the bone tissue of the regenerate was dominated by osteocytes with long processes, and in the second group of animals – with short ones (fig. 2).
In ultrathin sections of regenerate of the animals of both experimental groups, osteoblasts were mainly found, the cytoplasmic membrane of which formed short processes, and clusters of collagen fibers were detected in the adjacent intercellular substance. In osteoblasts of animals of the first group, the perinuclear spaces were not expanded, granules of condensed chromatin were concentrated along the periphery of the nucleus, and the matrix retained an average electron density. Mitochondria with a large number of cristae and a fine-grained matrix of medium electron density were detected in the cytoplasm. In osteoblasts, hyperplasia of the granular endoplasmic reticulum was also detected, and its cisterns were flattened and filled with a non-fibrous substance of medium electron density. Numerous ribosomes were located on the membranes of the endoplasmic reticulum and in the cytoplasm. Golgi’s lamellar cytoplasmic complex had a typical structure and localization in the cytoplasm, and its smooth membranes were stacked. But in animals of the second group, the nuclei of osteoblasts had deep intaginations, chromatin, which was localized along the nuclear membrane in the form of an osmiophilic ring. The perinuclear spaces were not expanded. The mitochondria of osteoblasts had a rounded shape, the matrix – a coarse-fiber structure with areas of electronic transparency, partially destroyed by cristae. A small number of free-lying ribosomes and ribosomes, which were associated with endoplasmic reticulum membranes, were also observed in the cytoplasm. In this case, the granular endoplasmic reticulum represented a system of electron-transparent vacuoles of various shapes and sizes (fig. 2).
Discussion
The study using scanning electron microscopy found that in the area of the bone defect on the 3rd day after its application, the rats of both groups showed signs of traumatic inflammation. The latter consisted in the presence of polymorphism of blood cells and connective tissue cell elements that provided the formation of granulation tissue. At the same time, we note that no differences between the 2 groups of animals during this period of the experiment were found using scanning electron microscopy. However, using transmission electron microscopy, it was found that the intake of excessive amounts of lead and cadmium in the body led to a decrease in the biosynthetic activity of lymphocytes, as well as the appearance of dystrophic and destructive changes in them. The latter was evidenced by the presence in the regenerate lymphocytes of a small number of cisterns of granular endoplasmic reticulum, a strongly expanded perinuclear space and an enlightened cytoplasm.
On the 24th day after the injury, using scanning electron microscopy, it was found that osteoblasts and osteocytes appeared on the surface of trabeculae and in the lacunae of bone tissue of regenerate of the animals of both experimental groups. Osteocytes in animals of the 1st group had mainly long, and in animals of the second group short processes. It should be noted that the size of the processes of osteocytes is one of the important morphological signs of their maturity. Thus, it is known that mature (secondary) osteocytes have long processes and are located in lacunae of lamellar bone tissue, while immature (primary) osteocytes of coarse-fibrous bone tissue are characterized by short processes [2]. Thus, in the last period of the experiment, primary or immature osteocytes prevailed in the bone tissue of regenerate of the animals of the 2nd group. In turn, Lu H et al, examining the distal epiphysis of the thighs of rats with scanning electron microscopy after ingesting excessive amounts of lead and cadmium, indicated a decrease in the size of bone lacunae, but did not report the cellular elements that are located there [27]. We also note that there are works in the scientific literature devoted to the excessive influence of lead and/or cadmium on the ultrastructure of cellular elements of bone tissue. However, such studies were conducted in conditions of physiological regeneration of bone tissue in the epiphyses, as well as on cultured osteoblasts of the skull bones. So, for example, Lu H et al using transmission electron microscopy found that when the rats received an excessive amount of lead and cadmium combination to the osteoblasts of the femoral epiphysis, there was a decrease in the number of organelles, focal cytolysis, as well as condensation and marginalization of chromatin in combination with a greater more floc [27]. According to the data of Zhao H and co-authors, mitochondria were changed in the conditions of 24-hour exposure to an increased concentration of cadmium alone in cultured osteoblasts of rat skull bones [30]. Thus, after Cd treatment at a dose of 1 µM, swollen mitochondria with featureless crystals were observed in osteoblasts, and an increase in the Cd dose from 2 to 5 µM led to the disappearance of mitochondrial crystals and the appearance of cytoplasmic vacuolation. In turn, in the presence of an excess of lead alone, Bonucci E et al found that the greatest changes in the tibial and femoral bones of Collie puppies were exposed to osteoclast nuclei, which were subjected to pyknosis and contained inclusion bodies with a high content of lead [31]. At the same time, osteoblasts and osteocytes did not contain inclusion bodies, since lead does not reach a high enough concentration in these cells. In our study, under the conditions of ingestion of excessive amounts of lead and cadmium into the body of rats, signs of dystrophic and destructive changes were observed in osteoblasts of regenerate bone tissue. The latter was evidenced by the presence in the nuclei of osteoblasts of an electron-transparent matrix, numerous intussusceptions of nuclear membrane, partially destroyed cristae, coarse-fiber structure and areas of electronic transparency of the matrix of mitochondria, as well as electron-transparent cisterns of the granular endoplasmic reticulum.
Conclusion
Subchronic receipt of excessive amounts of lead and cadmium in the body leads to the occurrence of dystrophic and destructive changes in the cellular elements of bone regenerate and slowing their maturation.
Oleksii Korenkov, MD | o.koren'kov@med.sumdu.edu.ua | www.int.sumdu.edu.ua
Zdroje
- Pepa GD, Brandi M. Microelements for bone boost: the last but not the least. Clin Cases Miner Bone Metab 2016; 13(3): 181–185. Available on DOI: <https://doi.org/10.11138/ccmbm/2016.13.3.181>.
- Omelyanenko NP. Histophysiology, Biochemistry, Molecular Biology. CRC Press: Boca Raton, Florida, USA 2013.
- Zofkova I, Davis M, Blahos J. Trace elements have beneficial, as well as detrimental effects on bone homeostasis. Physiol Res 2017; 66(3): 391–402. Available on DOI: <https://doi.org/10.33549/physiolres.933454>.
- Roberts JL, Drissi H. Advances and Promises of Nutritional Influences on Natural Bone Repair. J Orthop Res 2020; 38(4): 695–707. Available on DOI: <https://doi.org/10.1002/jor.24527>.
- Nechytailo L, Danyliv S, Kuras L et al. Dynamics of changes in cadmium levels in environmental objects and its impact on the bio-elemental composition of living organisms. Braz J Biol 2023; 84: e271324. Available on DOI: <https://doi.org/10.1590/1519–6984.271324>.
- Priya PS, Nandhini PP, Arockiaraj J. A comprehensive review on environmental pollutants and osteoporosis: Insights into molecular pathways. Environ Res 2023; 237(Pt 2): 117103. Available on DOI: <https://doi.org/10.1016/j.envres.2023.117103>.
- Rodríguez J, Mandalunis PM. A Review of Metal Exposure and Its Effects on Bone Health. J Toxicol 2018; 2018: 4854152. Available on DOI: <https://doi.org/10.1155/2018/4854152>.
- Vasilenko TO, Merciful RO, Masyuk DM et al. Sanitary and Toxicological assessment of drinking water of agricultural enterprises for the content of heavy metals. Bulletin of Sumy national agrarian University. Series “Animal Husbandry” 2017; 5/2(32): 20–26.
- Lu HK, Dai SJ, Yin ZQ, Yuan GP et al. Study on damage of bone in rat induced by experimental acute combined exposure to lead and cadmium. Chin Vet Sci 2012; 42(12): 1278–1282.
- Carmouche JJ, Puzas JE, Zhang X et al. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspect 2005; 113(6): 749–755. Available on DOI: <https://doi.org/10.1289/ehp.7596>.
- Tarasco M, Cardeira J, Viegas NM et al. Anti-Osteogenic Activity of Cadmium in Zebrafish. Fishes 2019; 4(1): 11. Available on DOI: <https://doi.org/10.3390/fishes4010011>.
- Gur E, Waner T, Barushka-Eizik O et al. Effect of cadmium on bone repair in young rats. J Toxicol Environ Health 1995; 45(3): 249–260. Available on DOI: <https://doi.org/10.1080/15287399509531994>.
- Tang L, Chen X, Bao Y et al. CT Imaging Biomarkers of Bone Damage Induced by Environmental Level of Cadmium Exposure in Male Rats. Biol Trace Elem Res 2016; 170(1): 146–151. Available on DOI: <https://doi.org/10.1007/s12011–015–0447–8>.
- Browar AW, Leavitt LL, Prozialeck WC et al. Levels of Cadmium in Human Mandibular Bone. Toxics 2019; 7(2): 31–36. Available on DOI: <https://doi.org/10.3390/toxics7020031>.
- Nawrot T, Geusens P, Nulens TS et al. Occupational cadmium exposure and calcium excretion, bone density, and osteoporosis in men. J Bone Miner Res 2010; 25(6): 1441–1445. Available on DOI: <https://doi.org/10.1002/jbmr.22>.
- Dahl C, Søgaard AJ, Tell GS et al. Do cadmium, lead, and aluminum in drinking water increase the risk of hip fractures? A NOREPOS study. Biol Trace Elem Res 2014; 157(1): 14–23. Available on DOI: <https://doi.org/10.1007/s12011–013–9862-x>.
- Wallin M, Barregard L, Sallsten G et al. Low-Level Cadmium Exposure Is Associated With Decreased Bone Mineral Density and Increased Risk of Incident Fractures in Elderly Men: The MrOS Sweden Study. J Bone Miner Res 2016; 31(4): 732–741. Available on DOI: <https://doi.org/10.1002/jbmr.2743>.
- Winiarska-Mieczan A. Protective effect of tea against lead and cadmium-induced oxidative stress – a review. Biometals 2018; 31(6): 909–926. Available on DOI: <https://doi.org/10.1007/s10534–018–0153-z>.
- Wang X, Bao R, Fu J. The Antagonistic Effect of Selenium on Cadmium-Induced Damage and mRNA Levels of Selenoprotein Genes and Inflammatory Factors in Chicken Kidney Tissue. Biol Trace Elem Res 2018; 181(2): 331–339. Available on DOI: <https://doi.org/10.1007/s12011–017–1041-z>.
- Berroukche A, Labani A, Terras MM. Antagonist effects of cadmium and zinc on the histological structures of the lungs, liver and kidneys in Wistar rats. Environnement Risques Sante 2015; 14(2): 163–171. Available on DOI: <https://doi.org/10.1684/ers.2015.0772>.
- Huang X, Liu T, Zhao M et al. Protective Effects of Moderate Ca Supplementation against Cd-Induced Bone Damage under Different Population-Relevant Doses in Young Female Rats. Nutrients 2019; 11(4): 849. Available on DOI: <https://doi.org/10.3390/nu11040849>.
- Rani A, Kumar A, Lal A et al. Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 2014; 24(4): 378–399. Available on DOI: <https://doi.org/10.1080/09603123.2013.835032>.
- Patra RC, Rautray AK, Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 2011; 2011: 457327. Available on DOI: <https://doi.org/10.4061/2011/457327>.
- Al-Ghafari A, Elmorsy E, Fikry E et al. The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress. PLoS One 2019; 14(11): e0225341. Available on DOI: <https://doi.org/10.1371/journal.pone.0225341>.
- Beier EE, Sheu TJ, Dang D et al. Heavy metal ion regulation of gene expression: mechanism by which lead inhibits osteoblastic bone-forming activity through modulation on the Wnt/β-catenin signaling pathway. J Biol Chem 2015; 290(29): 18216–18226. Available on DOI: <https://doi.org/10.1074/jbc.M114.629204>.
- Sun K, Mei W, Mo S et al. Lead exposure inhibits osteoblastic differentiation and inactivates the canonical Wnt signal and recovery by icaritin in MC3T3-E1 subclone 14 cells. Chem Biol Interact 2019; 303: 7–13. Available on DOI: <https://doi.org/10.1016/j.cbi.2019.01.039>.
- Lu H, Yuan G, Yin Z et al. Effects of subchronic exposure to lead acetate and cadmium chloride on rat’s bone: Ca and Pi contents, bone density, and histopathological evaluation. Int J Clin Exp Pathol 2014; 7(2): 640–647.
- Yuan G, Lu H, Yin Z et al. Effects of mixed subchronic lead acetate and cadmium chloride on bone metabolism in rats. Int J Clin Exp Med 2014; 7(5): 1378–1385.
- Lopes RH, Silva CRDV, Salvador PT et al. Surveillance of Drinking Water Quality Worldwide: Scoping Review Protocol. Int J Environ Res Public Health 2022; 19(15): 8989. Available on DOI: <https://doi.org/10.3390/ijerph19158989>.
- Zhao H, Liu W, Wang Y et al. Cadmium induces apoptosis in primary rat osteoblasts through caspase and mitogen-activated protein kinase pathways. J Vet Sci 2015; 16(3): 297–306. Available on DOI: <https://doi.org/10.4142/jvs.2015.16.3.297>.
- Bonucci E, Barckhaus RH, Silvestrini G et al. Osteoclast changes induced by lead poisoning (saturnism). Appl Pathol 1983; 1(5): 241–250.
Štítky
Biochemie Dětská gynekologie Dětská radiologie Dětská revmatologie Endokrinologie Gynekologie a porodnictví Interní lékařství Ortopedie Praktické lékařství pro dospělé Radiodiagnostika Rehabilitační a fyzikální medicína Revmatologie Traumatologie OsteologieČlánek vyšel v časopise
Clinical Osteology

2023 Číslo 4
Nejčtenější v tomto čísle
- Calcium metabolism and its disorders: hypercalcemia and hypocalcemia
- Effect of toxic metals on the bone regeneration
- Latest research and news in osteology
- Effects of selected heavy metals on the metabolism and healing processes of craniofacial bones
